*On June 1, 2023 Emerson’s Climate Technologies business became a new standalone company – Copeland. Though our name has changed, we are building on more than a century of HVACR innovation and industry leadership, and Copeland continues to offer the same products, industry stewardship, and learning opportunities you’ve grown to trust. Information found on this webpage posted before June 1, 2023 may contain our old name or branding, but you can be at ease knowing it was created with the knowledge and expertise of Copeland.
This is post three of CO2 as a Refrigerant, a blog series covering the fundamental considerations and characteristics of CO2 as a refrigerant (R-744).
Carbon dioxide (the element CO2) is a naturally occurring substance in Earth’s atmosphere comprised of approximately 0.04 percent CO2 (or 417 parts per million [ppm]). It is produced during respiration by most living organisms and absorbed by plants. Today, the burning of fossil fuels is the primary cause of increased CO2 concentration in the atmosphere.
R-744 is a byproduct of the industrial manufacture process during the production of ammonia, alcohol and fertilizer. The gas is captured, purified and compressed in a multi-step process — and finally liquefied for use as a refrigerant.
In its processed state as a refrigerant (R-744), its thermophysical characteristics differ greatly from traditional refrigerants. Namely, R-744 has a higher triple point and lower critical point than hydrofluorocarbon (HFC) refrigerants. Figure 1 demonstrates the occurrence of CO2’s triple and critical points on a phase change diagram.
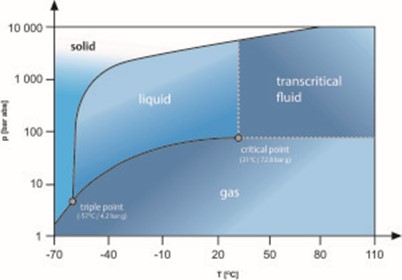
Figure 1: R-744/CO2 phase change diagram
The triple point occurs at 60.9 psig (4.2 bar) and -69.8 °F (-56.6 °C). The liquid phase of the refrigerant ceases to exist below this point, as the refrigerant transforms into dry ice. At atmospheric pressure of 0 psig (0 bar), solid R-744 sublimes directly to a gas. Dry ice produces 845 times its volume in gas at 59 °F (15 °C) and 1 atm (e.g., 1 oz. of dry ice will produce 845 oz. of CO2 vapor as it sublimes.) Solid R-744 has a surface temperature of -109.3 °F (-78.5 °C). If R-744 is at a pressure above its triple point and the pressure is reduced to below the triple point (i.e., to atmospheric pressure), it will transform directly to a solid (i.e., dry ice). This can occur when charging an evacuated refrigeration system with liquid R-744. Dry ice can also occur in the system piping anytime the internal pressure is below 62.4 psig (4.2 bar).
The critical point occurs at 87.8 °F (31 °C), which is typically below the maximum design condensing temperatures for most climate zones. Above the critical point, when the temperature and pressure are at or above 87.8 °F (31 °C) and 1,055 psig (72.7 bar), respectively, CO2 exists as a supercritical fluid. A phase change cannot occur when heat is removed from a supercritical fluid. Thus, when a CO2 booster system is operating in supercritical mode, R-744 will not condense. The pressure or temperature must be reduced below its critical point for liquidation to occur.
The critical point is the condition at which the liquid and gas densities are the same. Above this point, distinct liquid and gas phases do not exist.
The triple point is condition at which sold, liquid and gas co-exist.
No other commonly used refrigerant has such a low critical temperature. As a result, other refrigerants always condense as heat is removed on the high side of systems.
The boundaries of the supercritical fluid region are:
- The critical temperature 87.8 °F (31 °C) to the sub-cooled liquid region
- The critical pressure 1,055.9 psig (72.8 bar) to the superheated gas region
Table 1 compares the basic properties of R-744 with other refrigerants commonly used in the retail sector.
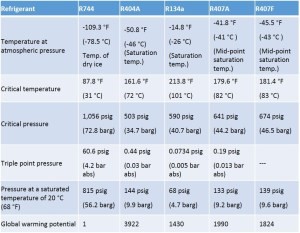
Table 1: Basic properties of R-744 compared with other refrigerants. *GWP values are from the Intergovernmental Panel on Climate Change, 4th assessment report
Figure 2 shows the pressure enthalpy chart of the critical point and the range of the supercritical fluid region.
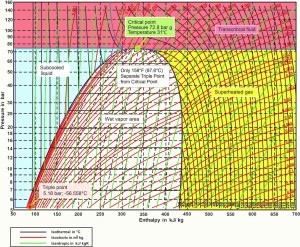
Figure 2: Pressure enthalpy chart for R-744
R-744’s higher operating pressures presented early challenges in CO2 applications compared to other commercial refrigerants. For perspective, Figure 3 compares the relative pressures of R-744 with R-404A and R-134A.
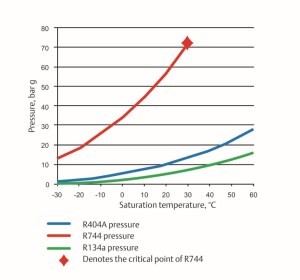
Figure 3: Pressure-temperature relationship comparison
Note that the saturation curve for R-744 does not extend beyond 87.8 °F (31 °C) because this is its critical point. Above this condition, there is no distinction between liquid and gas. Operation above this pressure is known as supercritical operation which we will discuss in the next post of this CO2 as a Refrigerant series.

Copeland Aligns its Family of Brands for the Future
As a critical milestone in our journey as a standalone company, Copeland is excited to unveil a...

Facility Health Score Insights Program Transforms Enterprise Maintenance
Leveraging refrigeration performance data drives food retail cost reductions. Maintaining proper...

State-level decarbonization efforts ramp up
New York continues its climate policies in the face of federal deregulation. I recently returned...